Clinical Pharmacology in Vaccine Development/COVID-19/RSV
101: The SARS-CoV-2 Mpro Inhibitor Ibuzatrelvir is a Substrate but not an Inducer nor Inhibitor of CYP3A
- SS
Siddhee A. Sahasrabudhe, MS, PhD
Manager Clinical Pharmacology
Pfizer Inc, Massachusetts, United States - SS
Siddhee A. Sahasrabudhe, MS, PhD
Manager Clinical Pharmacology
Pfizer Inc, Massachusetts, United States - BA
Bisrat Abraham, MD
Clinical Director
Pfizer Inc, New York, United States - NA
Niki Alami, MD
Vice President, Head of Anti-Infectives Clinical Research
Pfizer Inc, Massachusetts, United States - AB
Arthur Bergman, PhD
Sr Director, Clin Pharm Head
Pfizer Inc, Connecticut, United States - RG
Ruffy Guilatco, PhD
Sr Manager Biostatistics
Pfizer Inc, Mountain Province, Philippines - FH
Frances Hackman, PhD
Director, Statistics
Pfizer Inc, England, United Kingdom - MM
Mahta Mortezavi, MD
Clinical Director
Pfizer Inc, Pennsylvania, United States - MS
Mona Shahbazi, MS, NP
Clinical Research Clinician, Associate Director
Pfizer Inc, Connecticut, United States 
Ravi Shankar P. Singh, PhD
Senior Director
Pfizer Inc
Cambridge, Massachusetts, United States- ST
Sima Toussi, BS, MD
Senior Clinical Director
Pfizer, New York, United States
Presenting Author(s)
1st/Primary Author(s)
Co-Author(s)
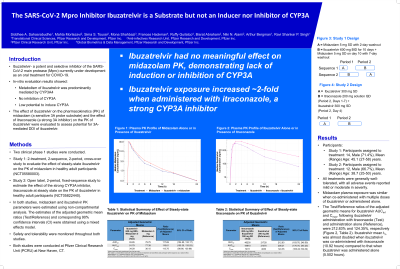
Ibuzatrelvir is a potent and selective inhibitor of the SARS-CoV-2 main protease (Mpro) currently under development as an oral treatment for COVID-19. In-vitro evaluations showed metabolism of ibuzatrelvir was predominantly mediated by CYP3A4 with no inhibition of CYP3A, and low potential to induce CYP3A. The effect of ibuzatrelvir on the pharmacokinetics (PK) of midazolam (a sensitive 3A probe substrate) and the effect of itraconazole (a strong 3A inhibitor) on the PK of ibuzatrelvir were evaluated to assess potential for 3A-mediated DDI of ibuzatrelvir.
Description of Methods & Materials:
The first study was a 2-treatment, 2-sequence, 2-period, cross-over study to evaluate the effect of steady-state ibuzatrelvir (600 mg, BID for 10 days) on the PK of midazolam (5 mg, SD) in healthy adult participants.
The second study was an open label, 2-period, fixed-sequence study to estimate the effect of the strong CYP3A inhibitor, itraconazole at steady state (200 mg, QD), on the PK of ibuzatrelvir (300 mg, SD) in healthy adult participants. Midazolam and ibuzatrelvir PK parameters were estimated using non-compartmental analysis. In both studies, the estimates of the adjusted geometric mean ratios (Test/Reference) and corresponding 90% confidence intervals (CI) were obtained using a mixed effects model with design specific fixed and random effects. Safety and tolerability were monitored throughout both studies.
Data & Results:
There were 12 participants who completed each of the two studies. Overall, there were no severe adverse events, clinically meaningful laboratory, vital sign or ECG changes in either study.
Midazolam plasma exposure was similar when co-administered with multiple doses of ibuzatrelvir or administered alone. The Test/Reference ratios of the adjusted geometric means (90% CI) for midazolam AUCinf and Cmax were 117.69% (104.80%, 132.17%) and 113.75% (98.86%, 130.89%), respectively, following midazolam co-administration with ibuzatrelvir (Test) as compared to midazolam alone (Reference).
The Test/Reference ratios of the adjusted geometric means (90% CI) for ibuzatrelvir AUCinf and Cmax, following ibuzatrelvir administration with itraconazole (Test) and administration alone (Reference), were 212.83% (183.76%, 246.50%) and 124.35% (100.22%, 154.29%), respectively. Ibuzatrelvir mean t½ was approximately double when it was co-administered with itraconazole (10.92 hours) compared to that when it was administered alone (5.50 hours).
Interpretation, Conclusion or Significance:
Midazolam exposure did not meaningfully change in the presence of ibuzatrelvir indicating no CYP3A perpetrator DDI potential of ibuzatrelvir. As a victim, AUCinf and Cmax of ibuzatrelvir increased by 113% and 24% respectively, when administered in the presence of a strong CYP3A inhibitor. In general, ibuzatrelvir demonstrated minimal CYP3A mediated DDI potential.
Disclosures:
Citations/References:
Additional Information/Authors: Additional Authors: Mortezavi M, Toussi SS, Shahbazi M, Hackman F, Guilatco R, Abraham B, Alami NN, Bergman A, Singh RSP

